Any content you receive is for information purposes only. Always conduct your own research. *Sponsored
Oragenics, Inc. (NYSE American: OGEN) Just Hit Krypton Street's Radar Heading Into Today's Session—Thursday, March 12, 2026
Don't Miss The Next Breakout—Get Real-Time Alerts Sent Directly
To Your Phone. Up To 10X Faster Than Email.
Full Coverage On (OGEN) is Starting Now
Take A Look At (OGEN) While It's Still Early…
March 12, 2026
Early Radar Flashing | See Why (OGEN) is Lighting Up Our Screen Right Now
Dear Reader, After today's profile tapped $4.98 in the early session, marking an approximate 27% overnight move before the bell even rang, we're now turning our attention to this next idea that just landed on our radar. Every year, traumatic brain injuries disrupt more lives in America than stroke, Alzheimer's disease, Parkinson's disease, multiple sclerosis, and ALS combined. Yet despite the scale of this growing medical burden, doctors still have no FDA-approved dr-ug specifically for concussions. That has left patients, families, athletes, and military personnel facing a serious treatment gap at the very moment rapid intervention matters most. It has also intensified the search for a therapy that can reach the brain quickly, directly, and safely. Now, one clinical-stage biotech company is working to answer that need in a way few others can. Oragenics, Inc. (NYSE American: OGEN) is advancing a proprietary intranasal delivery technology designed to bypass the blood-brain barrier and deliver treatment directly to the source of neurological injury. And this is just one of the reasons why (OGEN) is topping our watchlist this morning—Thursday, March 12, 2026. But keep in mind, (OGEN) has less than 5M shares listed as available to the public, according to MarketWatch. When companies like this have small public floats, the potential exists for big moves if demand begins to shift. In fact, (OGEN) just made an approximate 79% move over the last month, from around $.62 on February 12 to $1.11 this week, according to data available from Barchart. 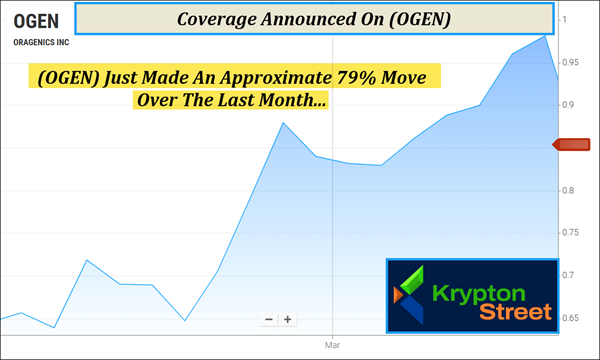
That underlying medical story is a major part of what makes the company worth watching. With no currently FDA-approved pharmacological treatments for concussions, the company is emerging as a notable name in the evolving field of brain health. Here's a closer look at how Oragenics, Inc. (NYSE American: OGEN)'s platform is designed to address that challenge. Revolutionizing Brain-Targeted Therapeutics
Oragenics, Inc. (NYSE American: OGEN) is a clinical-stage biotechnology company dedicated to fighting neurological conditions through its proprietary intranasal delivery platform. The company's lead candidate, ONP-002, is a dr-ug-device combination specifically engineered to deliver neuroprotective compounds directly to the brain via the nasal cavity. This method is designed to provide rapid therapeutic onset while minimizing systemic side effects, a critical factor when treating acute brain trauma. Traditionally, dr-ugs intended for the brain have struggled to cross the blood-brain barrier (BBB), often requiring high systemic doses that lead to unwanted toxicity in other organs. Oragenics aims to circumvent this hurdle entirely. The company is currently focused on the concussion and mild traumatic brain injury (mTBI) market, an area of medicine that has seen little innovation despite its prevalence in sports, military operations, and everyday accidents. Beyond its lead program, Oragenics believes its delivery technology has broad applications for other high-value indications, including Parkinson's disease, Alzheimer's disease, PTSD, and anxiety disorders. By utilizing a "nose-to-brain" route, the company aims to overcome the traditional challenges of oral or intravenous medications that struggle to reach the central nervous system effectively. This unique delivery mechanism could potentially transform how we treat a wide spectrum of chronic and acute neurological pathologies. Addressing a Large and Expanding Market
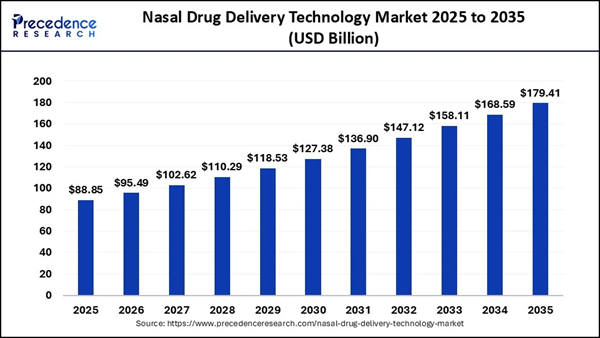
The scale of the nasal dr-ug delivery technology market is substantial, with projections suggesting it will grow from $95B in 2026 to more than $179B by 2035. Within this broader segment, the concussion market is projected to reach $8.58B by 2026 and expand to $10.43B by 2030. (OGEN) is focused on this projected $9B market through the advancement of ONP-002, which the company describes as the first and only clinical-stage pharmacological therapy for concussions and mTBI. With no standardized dr-ug-based treatment currently available for concussions, (OGEN) is working in an area of medicine that remains largely unaddressed. Recent operational milestones have also strengthened the company's forward path. On March 10, 2026, Oragenics announced that it received HREC approval to begin its Phase IIa clinical trial for ONP-002 in Australia. This clearance allows for patient enrollment across three clinical sites, marking an important step from preclinical development into human evaluation. In addition, the company has partnered with DUCK FLATS Pharma to support regulatory readiness for a future FDA Investigational New dr-ug (IND) application in the United States. That collaboration is intended to help align the clinical program with the standards required for a potential U.S. development pathway. Strategic Pipeline and Technical Positioning
The technical strength of (OGEN) is bolstered by its strategic partnership with Receptor.AI, an entity that uses artificial intelligence to accelerate pipeline development. This collaboration is intended to identify new therapeutic candidates that can utilize the company's intranasal device, effectively turning Oragenics into a multi-asset platform company rather than a single-product venture. By leveraging AI, the company can scan a vast number of chemical compounds to identify those most likely to treat neurodegeneration when delivered through its proprietary device. From a technical perspective, (OGEN) presents a unique profile with less than 5M shares listed as available to the public. In the world of biotechnology, a small float often means that positive clinical data or regulatory news can lead to rapid adjustments in valuation. Currently sitting around the $1 range, the company appears to be overlooked by the broader market despite its proximity to potential major clinical catalysts. The Q3 2025 shareholder update highlighted a clear vision: moving from the conceptual phase into full-scale execution and clinical validation. As we look at the landscape of 2026, the demand for effective brain health solutions has never been higher. Professional sports leagues, the military, and public health organizations are all searching for a way to mitigate the long-term effects of brain trauma, such as Chronic Traumatic Encephalopathy (CTE). (OGEN) is positioning ONP-002 as the answer to this call. By providing a portable, easy-to-use intranasal device, the company envisions a future where concussions are treated immediately on the sidelines or on the battlefield, potentially preventing the cascade of neurological damage that leads to permanent disability. The management team at (OGEN) has been methodically checking off regulatory boxes. From securing compliance on major exchanges to forming partnerships with top-tier pharmacological consultants, the infrastructure for a major clinical launch is now in place. The company's corporate presentation outlines a roadmap that includes not just the completion of the Australian trials, but a clear path toward global expansion. With the global nasal dr-ug delivery market expanding at a rapid clip, the timing for (OGEN)'s clinical progression couldn't be more fortuitous. 
7 Reasons Why (OGEN) is Topping Our Watchlist This Morning —Thursday, March 12, 2026…
1. Small Float: With fewer than 5M shares listed as available to the public according to MarketWatch, (OGEN)'s small float could have the potential for big moves if demand begins to shift. 2. Recent Momentum: (OGEN) just made an approximate 79% move over the last month, from around $.62 on February 12 to $1.11 this week 3. Unmet Need: Because there are currently no FDA-approved pharmacological treatments for concussions, (OGEN) is operating in a part of brain health that remains largely unaddressed. 4. Clinical Progress: After receiving HREC approval on March 10, 2026 to begin a Phase IIa clinical trial in Australia across three clinical sites, (OGEN) has moved its lead program into human evaluation. 5. Large Market: By focusing on concussion and mild traumatic brain injury, (OGEN) is targeting a projected $9B segment within the broader nasal delivery technology market expected to grow significantly over the coming decade. 6. AI Partnership: Through its collaboration with Receptor.AI, (OGEN) is using artificial intelligence to screen a large number of compounds and identify new candidates that may work with its proprietary intranasal device. 7. Regulatory Path: With support from DUCK FLATS Pharma to prepare a future FDA IND submission and a roadmap that includes global expansion, (OGEN) has been building the regulatory and operational framework needed for further clinical advancement. Take A Look At (OGEN) While It's Still Early…
As you can see, several key elements are now aligning around Oragenics, Inc. (NYSE American: OGEN). From a public float of fewer than 5M shares to a platform designed to bypass the blood-brain barrier, the company is working in an area of neuroscience that has seen limited progress despite the scale of concussion-related injuries. The recent HREC clearance allowing Phase IIa patient enrollment in Australia marks an important step as the program moves into human evaluation. At the same time, partnerships with Receptor.AI and DUCK FLATS Pharma highlight efforts to expand the pipeline and prepare for future regulatory filings. Taken together, these developments place (OGEN) at an interesting point as its clinical and operational milestones continue to unfold. We have all eyes on (OGEN) this morning—Thursday, March 12, 2026. Take a look at (OGEN) while it's still early. Also, keep a lookout for my next update, it could be hitting within the next 60-90 minutes. Sincerely, Alex Ramsay
Co-Founder / Managing Editor
Krypton Street Newsletter | 








_ims031226_128.png)
