Any content you receive is for information purposes only. Always conduct your own research. *Sponsored
Krypton Street Just Announced (ONCO) Will Be On Tomorrow's
Watchlist—Thursday, February 12, 2026
Don't Miss The Next Breakout—Get Real-Time Alerts Sent Directly
To Your Phone. Up To 10X Faster Than Email.
Keep Your Eyes Peeled—Full Coverage Will Go Live Early
In The Morning
Take A Look At (ONCO) Before Tomorrow Morning
February 11, 2026
On Deck For Tomorrow | See Why (ONCO) Just Hit Thursday's Radar Dear Reader, At Krypton Street, we've been watching a quiet shift accelerate in men's health: personalization is moving out of the lab and into real-world care, and it's starting to reshape how clinicians screen and decide what comes next. Prostate care is right in the center of that shift, as better testing and clearer decision support push the field toward fewer unnecessary procedures and more targeted next steps. That pressure is only rising as expectations climb for higher-accuracy screening and smarter treatment pathways. And the market backdrop helps explain why the spotlight keeps tightening: the global benign prostatic hyperplasia treatment market is forecasted to top $13B in 2026 and grow over 60% to exceed $21B by 2034, while the global prostate cancer diagnostics market is projected to top $10B in 2026 and grow around 70% to exceed $17B by 2034. 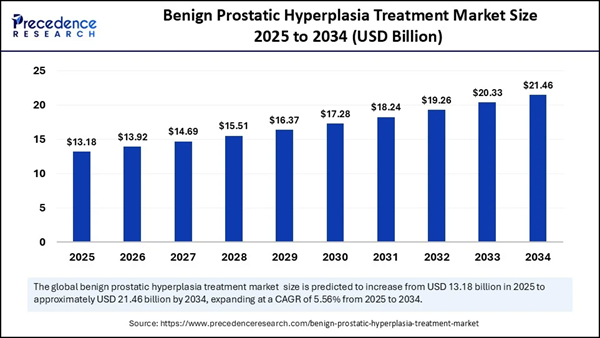
Onconetix, Inc. (Nasdaq: ONCO) is positioning itself within this growing market by focusing on prostate disease through a combination of therapeutics and diagnostics, using a dual-track strategy built to improve how prostate health decisions are made. And this is just one of the reasons why (ONCO) will be topping our watchlist tomorrow morning—Thursday, February 12, 2026. But keep in mind, (ONCO) has less than 1.3M shares listed as available to the public, according to MarketWatch. When companies have small floats like this, the potential exists for big moves if demand begins to shift. Right now, (ONCO) is sitting below $1.50 and appears to be flying under the radar of many screens. This is where things could get interesting fast. A Strategic Combination: The Blue Water + Proteomedix Roll-Up Story
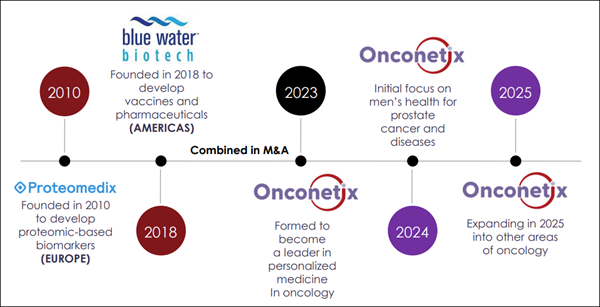
Onconetix, Inc. (Nasdaq: ONCO) is a commercial-stage biotechnology company headquartered in Cincinnati, Ohio, that was formed through the strategic combination of Blue Water Biotech and Proteomedix. Unlike many organizations that focus exclusively on either development or medical testing, (ONCO) operates a diversified portfolio that spans the entire patient journey. Their mission is to improve how prostate conditions—ranging from non-cancerous enlargement to aggressive malignancies—are identified, evaluated, and managed by clinicians. The company's distinctive value lies in its integrated approach. By housing both an FDA-approved therapeutic and a clinically validated diagnostic under one roof, (ONCO) aims to capture value at multiple points of the prostate disease pathway. This "personalized medicine" model is designed to reduce the high rate of unnecessary procedures and provide targeted relief for common symptoms, addressing a market that continues to grow as the global population ages. Recent milestones have transitioned the company from a developmental entity into an active commercial player. The commercial launch of their lead therapeutic product and the expansion of their diagnostic testing through major laboratory partnerships mark a significant turning point in the company's operational history. The Dual-Track Strategy: Therapeutics and Diagnostics
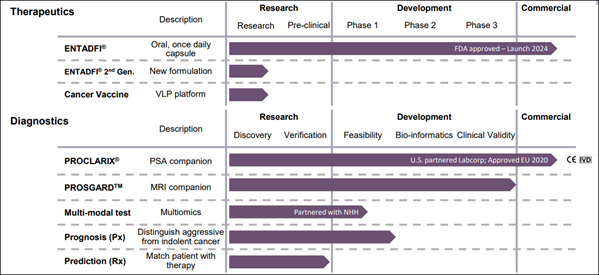
Addressing the BPH Burden with ENTADFI® The therapeutic pillar of (ONCO) is led by ENTADFI®, an FDA-approved, once-daily oral capsule for the treatment of benign prostatic hyperplasia (BPH). BPH is a non-cancerous enlargement of the prostate that affects a vast majority of men over age 50, often leading to debilitating urinary symptoms. ENTADFI® combines two established agents into a single capsule, and clinical data indicates it offers superior improvements in urinary flow and quality of life compared to finasteride alone. By simplifying the treatment regimen and enhancing efficacy, (ONCO) is targeting a massive primary care and urology market. Clarifying Cancer Decisions with PROCLARIX® On the diagnostic side, PROCLARIX® is a next-generation blood test designed to help doctors estimate the likelihood of clinically significant prostate cancer. It is specifically positioned as a companion to standard PSA testing, which is notorious for producing "gray zone" results that often lead to unnecessary and invasive biopsies. PROCLARIX® utilizes a proprietary algorithm to analyze blood markers, providing a risk score that helps clinicians decide whether a biopsy is truly necessary. The test is already CE-IVDR certified in Europe and is available in the U.S. as a lab-developed test through an exclusive partnership with Labcorp. Market Potential and Clinical Validation The market for prostate health is substantial: the benign prostatic hyperplasia (BPH) treatment market is estimated to top $13 B in 2026 and is projected to exceed $21 B by 2034, while the prostate cancer diagnostics market is forecast to top $10 B in 2026 and grow to more than $17 B by 2034. (ONCO) has secured a competitive edge through clinical validation, with PROCLARIX® being included in both European and American urology guidelines. This recognition is critical, as it supports the clinical utility of the test in cases where MRI results are uncertain. By reducing unnecessary biopsies, (ONCO) is not only improving patient outcomes but also addressing significant costs within the healthcare system. 7 Reasons Why (ONCO) Will Be Topping Our Watchlist Tomorrow —Thursday, February 12, 2026…
1. Under-Radar: Trending below $1.50, (ONCO) currently sits outside many common screening thresholds, which can limit visibility.
2. Small Float: With fewer than 1.3M shares available to the public, (ONCO)'s small float could see the potential for big moves if demand begins to shift. 3. Growing End Markets: The core areas tied to (ONCO) align with prostate health segments forecasted to reach $13B–$10B in 2026 and expand more than 60%–70% by 2034. 4. Guideline Inclusion: PROCLARIX® from (ONCO) is referenced in both European and American urology guidelines, supporting its real-world clinical relevance in uncertain cases. 5. Commercial Stage: Recent milestones have moved (ONCO) beyond development, with active commercial rollout of its lead therapeutic and expanded diagnostic availability. 6. Dual Platform: Unlike single-focus peers, (ONCO) combines a therapeutic product and a diagnostic test, allowing participation across multiple stages of prostate care. 7. Near-Term Trend Watch: Technical indicators for (ONCO) show it now trending above its 5-day moving average, which could suggest a possible shift in near-term momentum. Take A Look At (ONCO) Before Tomorrow Morning
Onconetix, Inc. (Nasdaq: ONCO) is no longer a "story" company; it is an execution company. With the foundational work of clinical trials and regulatory clearances largely complete, the focus has shifted entirely to commercial scale. The partnership with Labcorp for PROCLARIX® and the rollout of ENTADFI® represent the two primary engines that are expected to drive the company's valuation in the coming quarters. The medical community is increasingly leaning toward "active surveillance" and precision testing to avoid the complications of over-treating prostate conditions. ONCO is positioned directly in the path of this trend, offering tools that help identify which patients need surgery and which can be managed with oral therapies. For those keeping an eye on little-known companies at the intersection of oncology and personalized medicine, (Nasdaq: ONCO) provides an exciting case study in how biotechnology can evolve to meet modern clinical demands. As the company continues to report on its commercial progress and European pilot programs, Onconetix, Inc. (Nasdaq: ONCO) remains one under the radar company we're keeping on our watchlist. We'll have (ONCO) up on our screen early tomorrow morning. Take a look at (ONCO) tonight so you're ready before Thursday's session. And keep an eye out for my next report, it could be hitting earlier than usual. Sincerely, Alex Ramsay
Co-Founder / Managing Editor Krypton Street Newsletter
| 



0 التعليقات:
إرسال تعليق